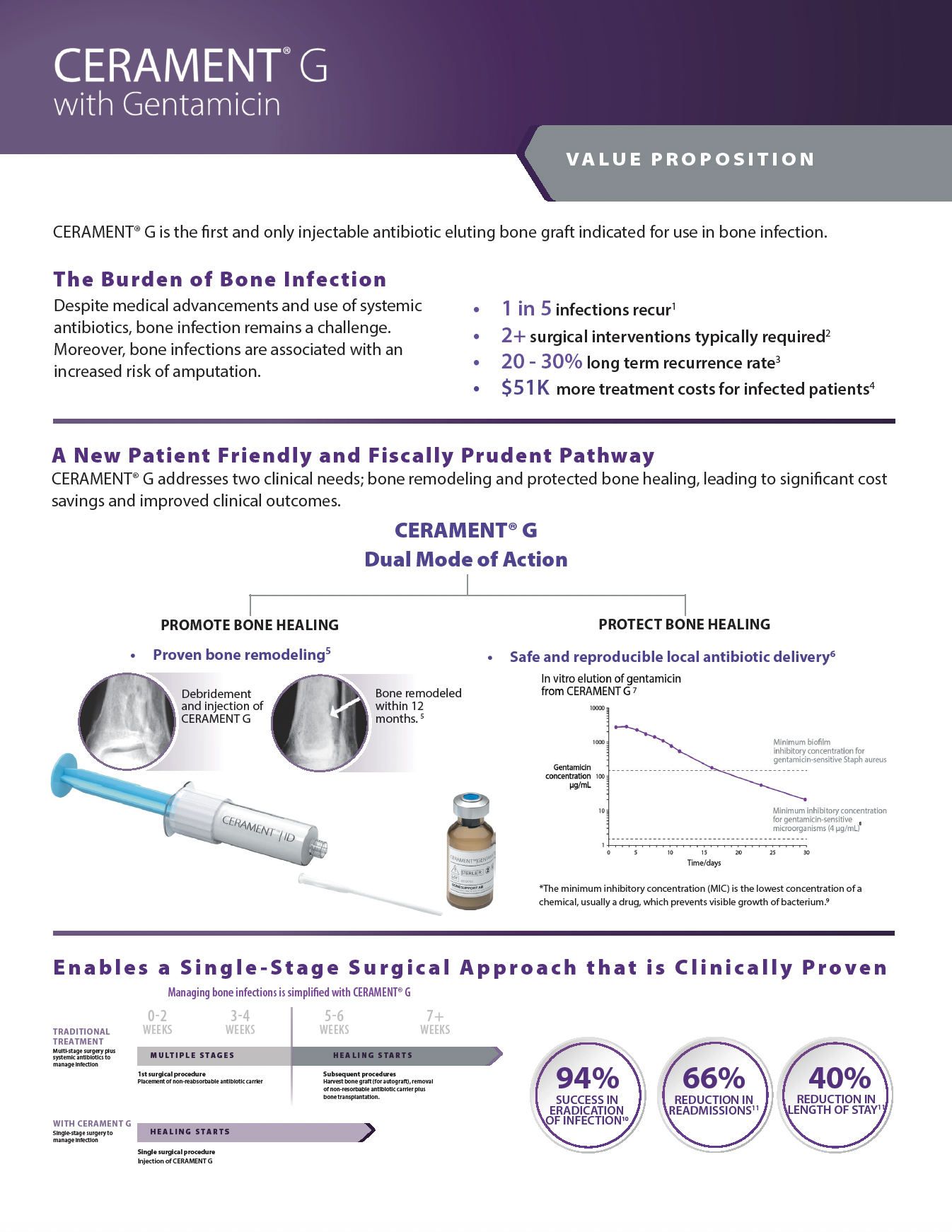
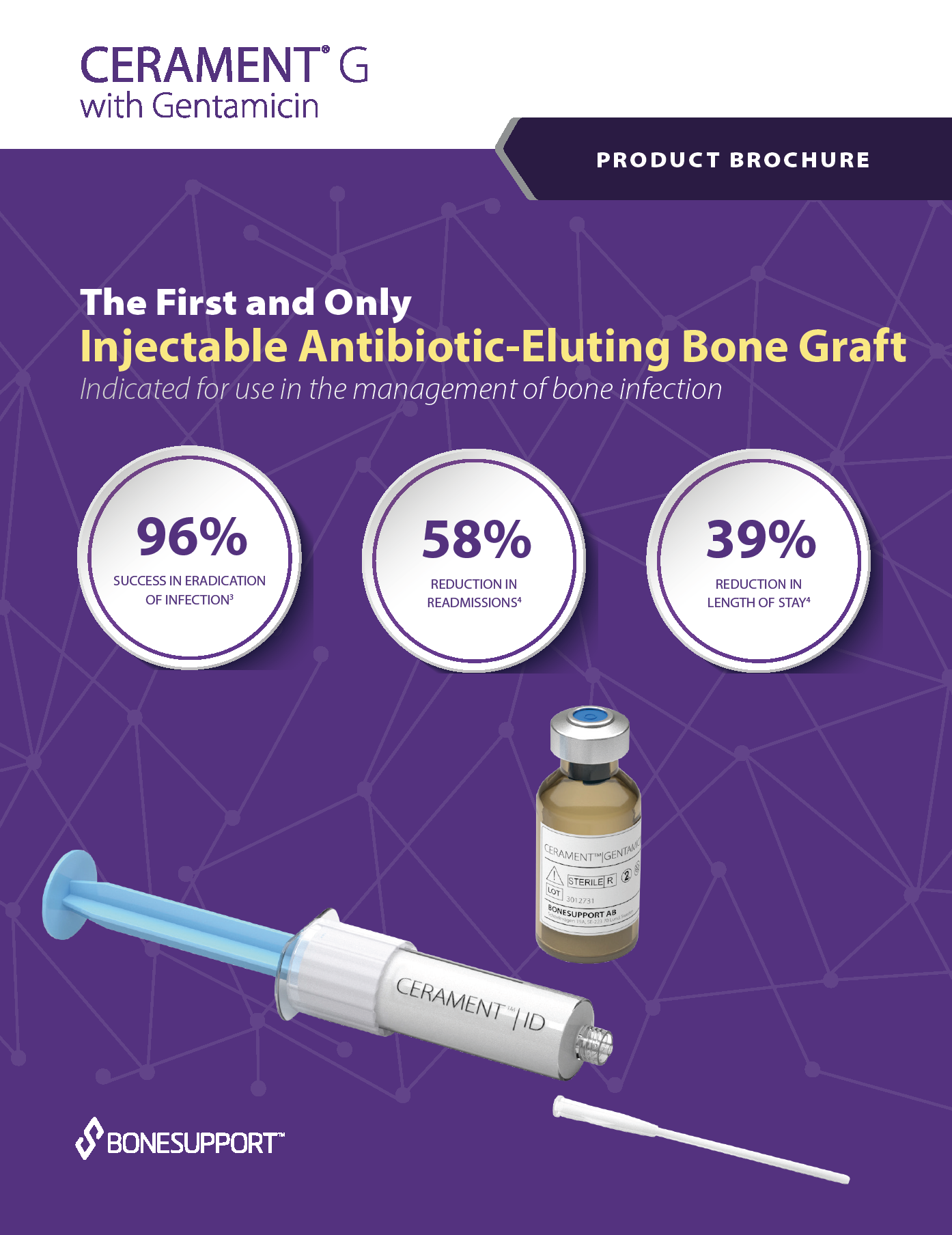
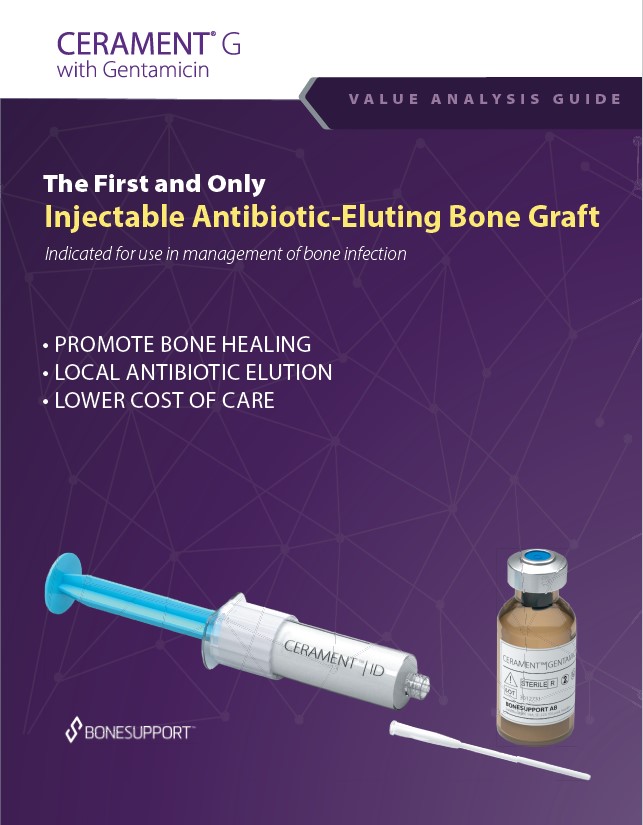
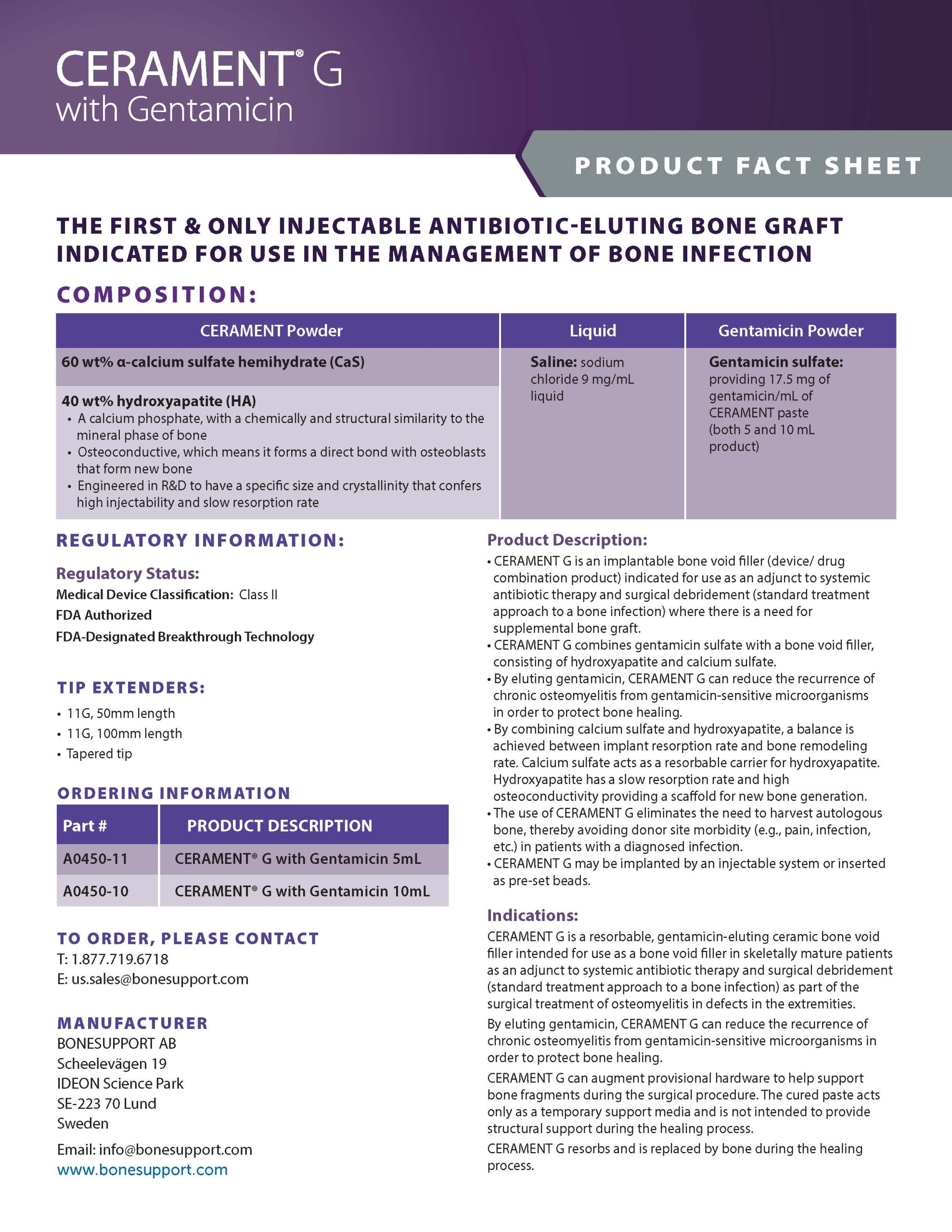
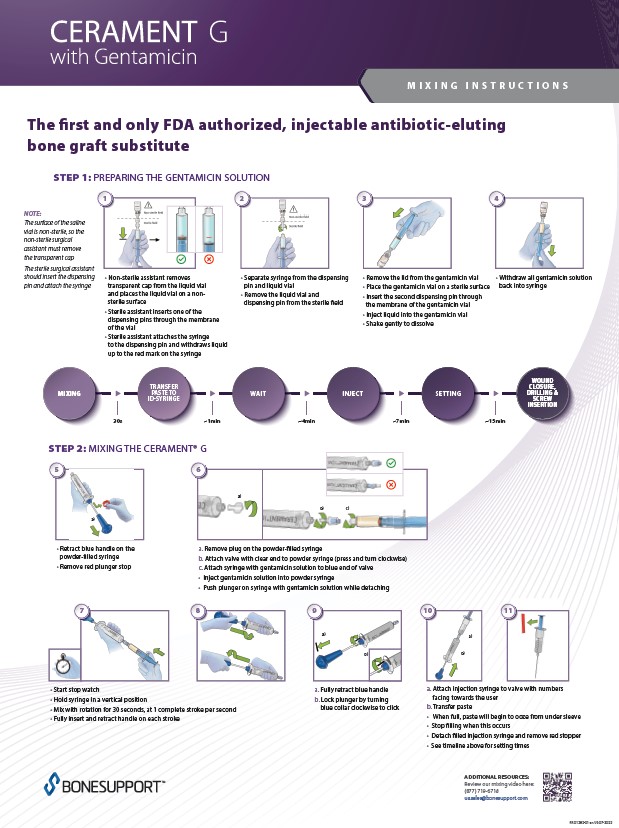
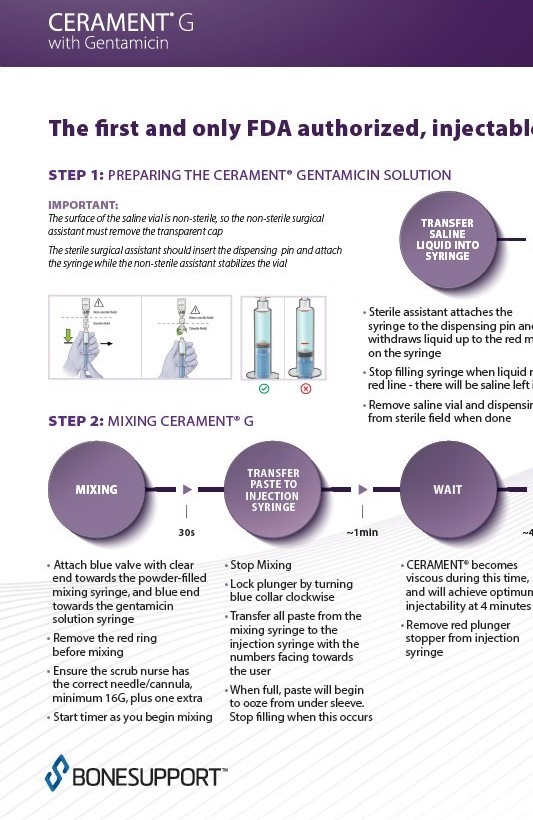
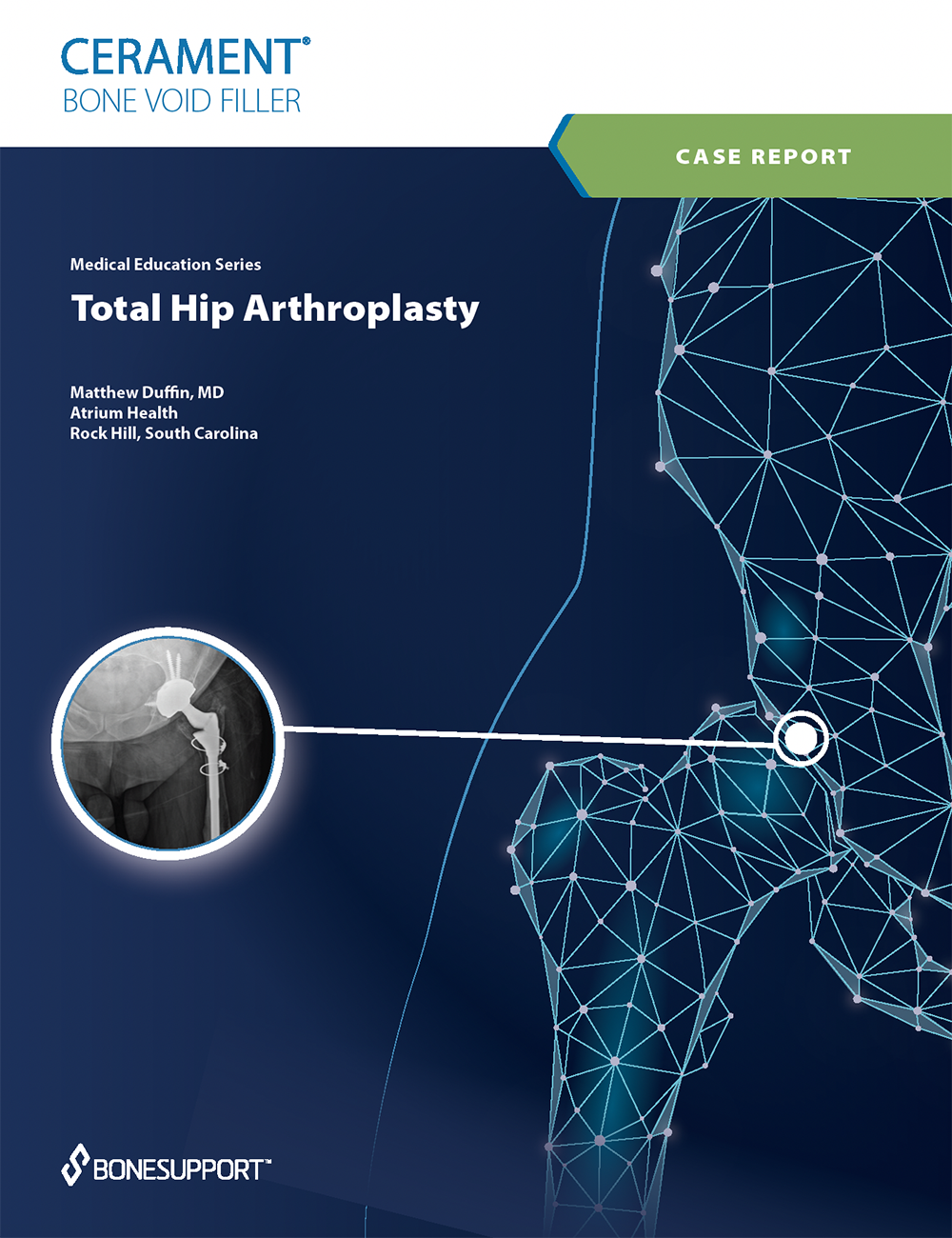
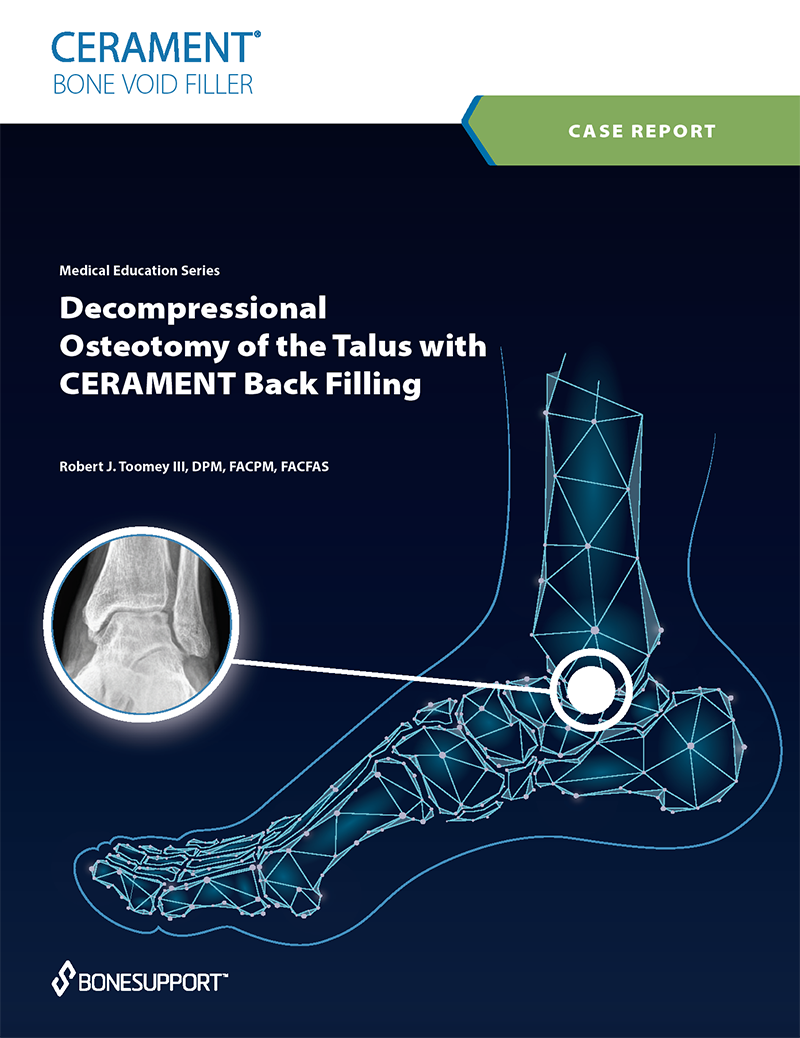
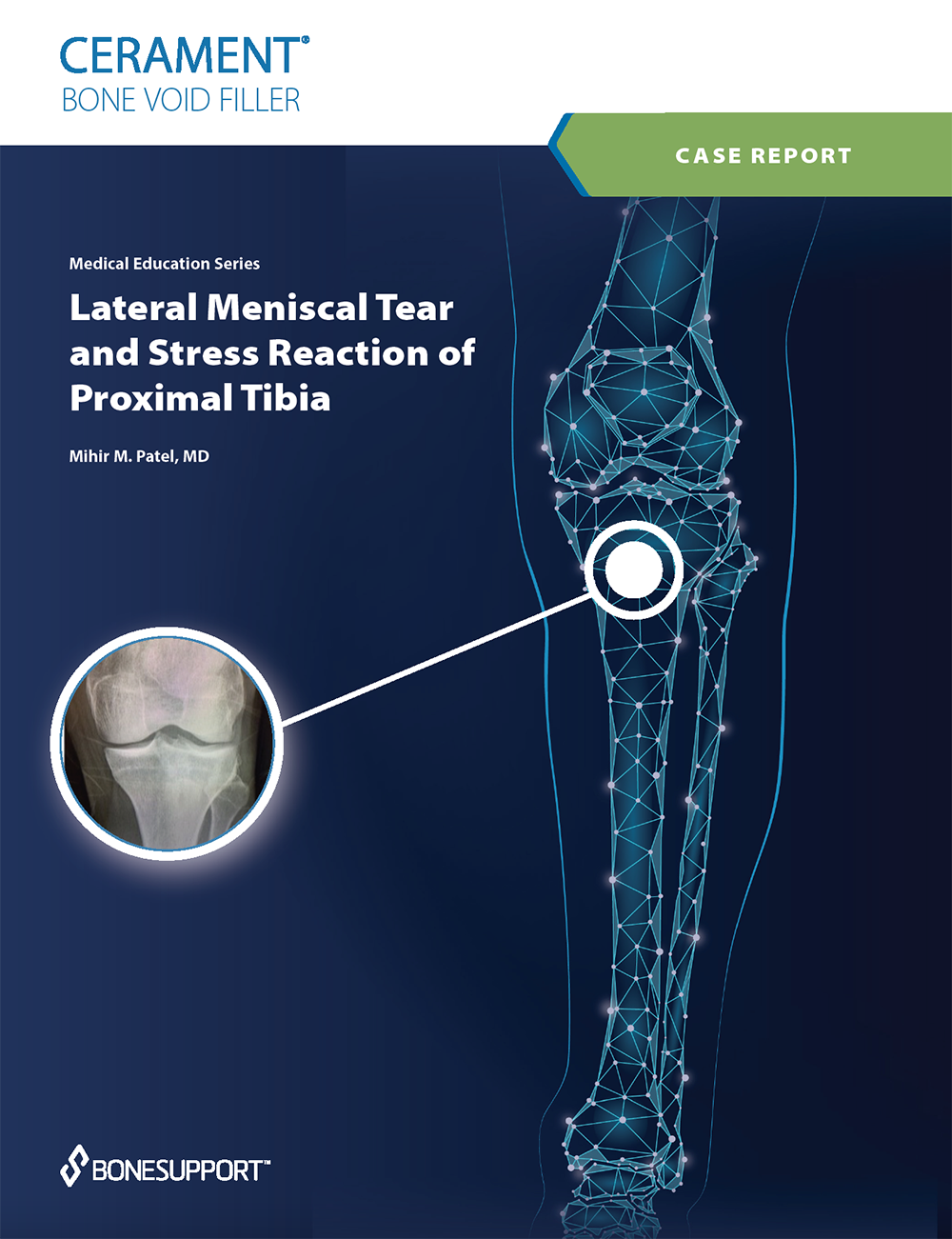
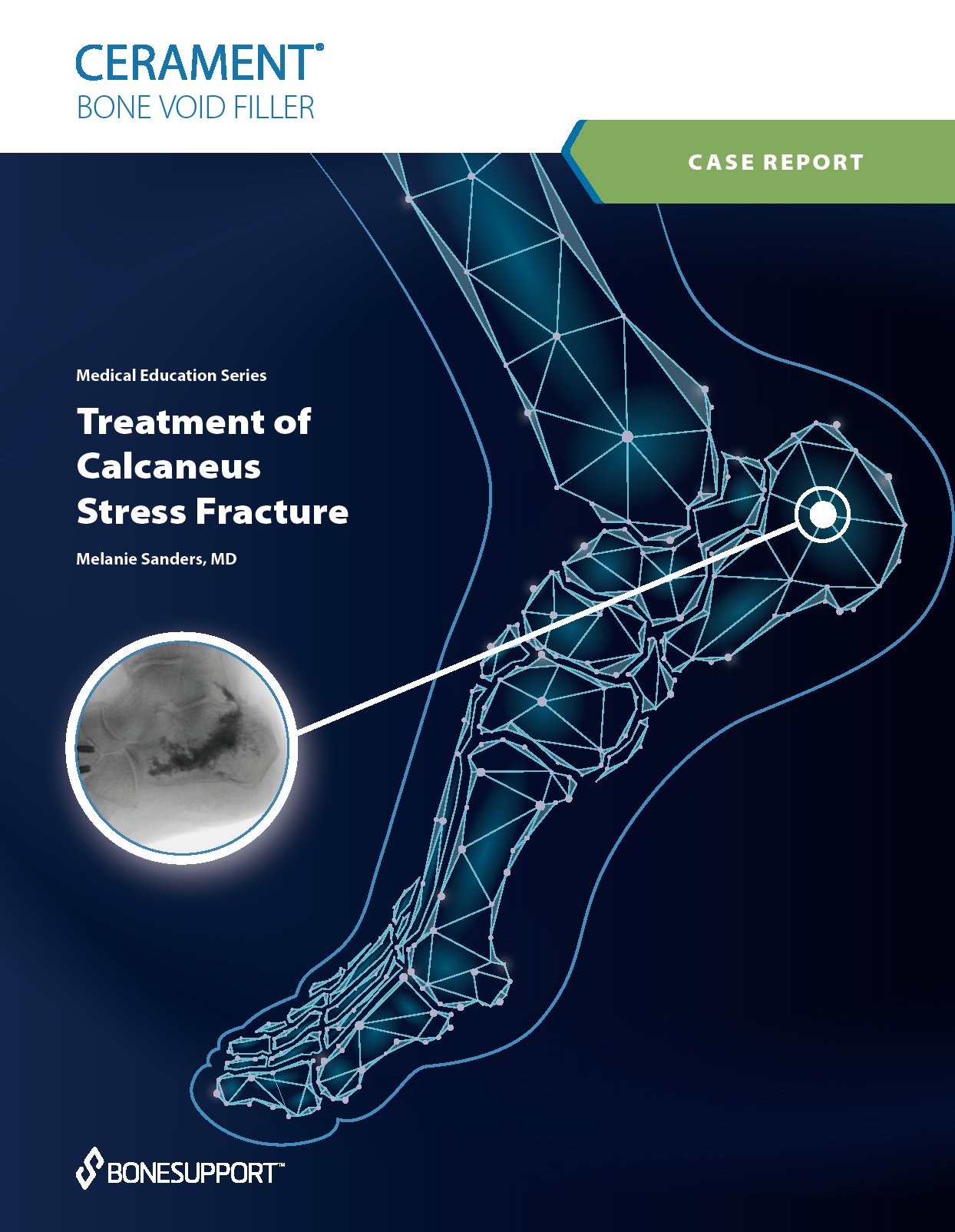
There are over 240+ publications and abstracts relating to CERAMENT®, and below are a selection to download. If you would like a full list, please email .
BONESUPPORT regularly holds educational webinars, covering topics such as osteomyelitis, diabetic foot, limb reconstruction, limb salvage and benign bone tumors. You can see our full list of webinars here.