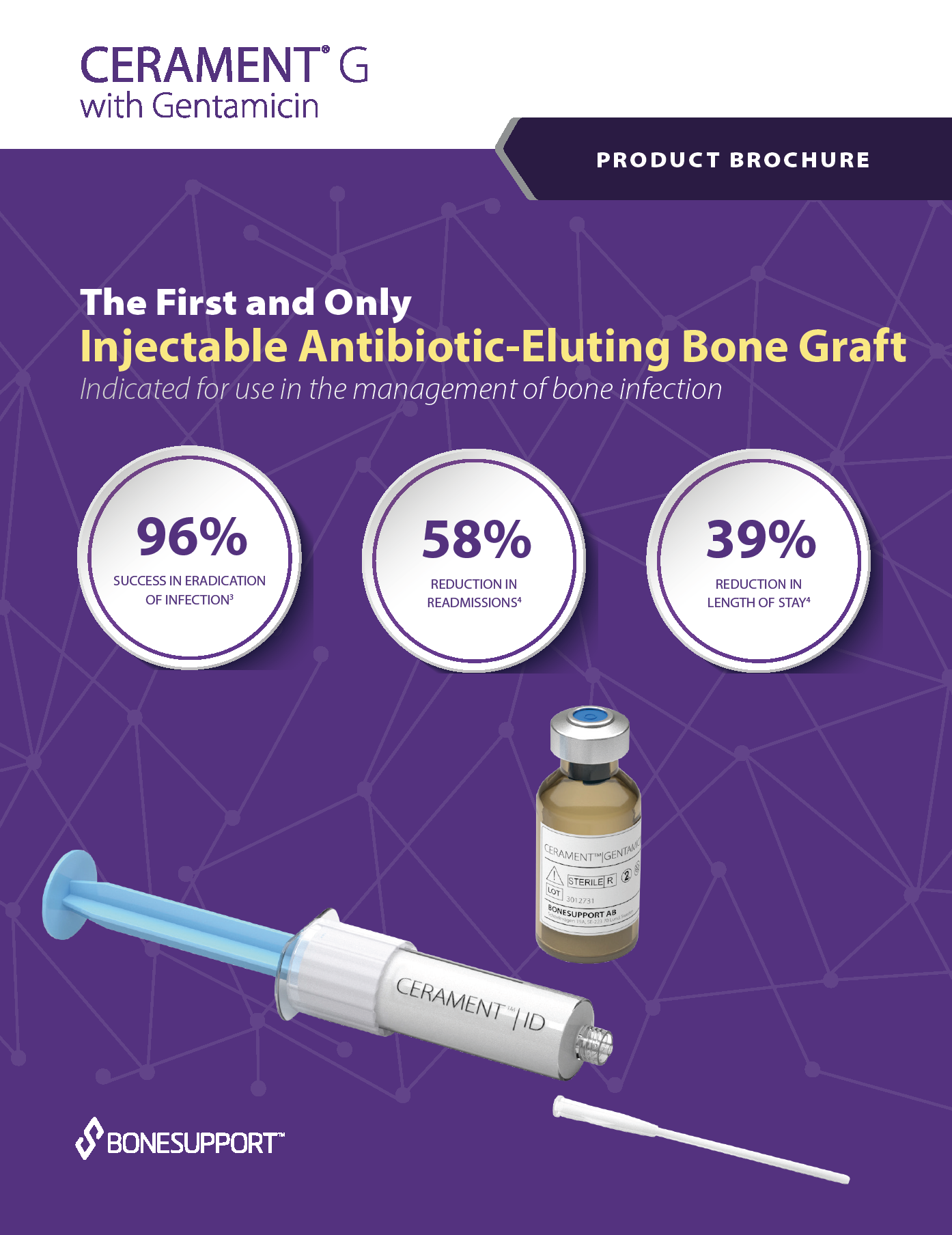
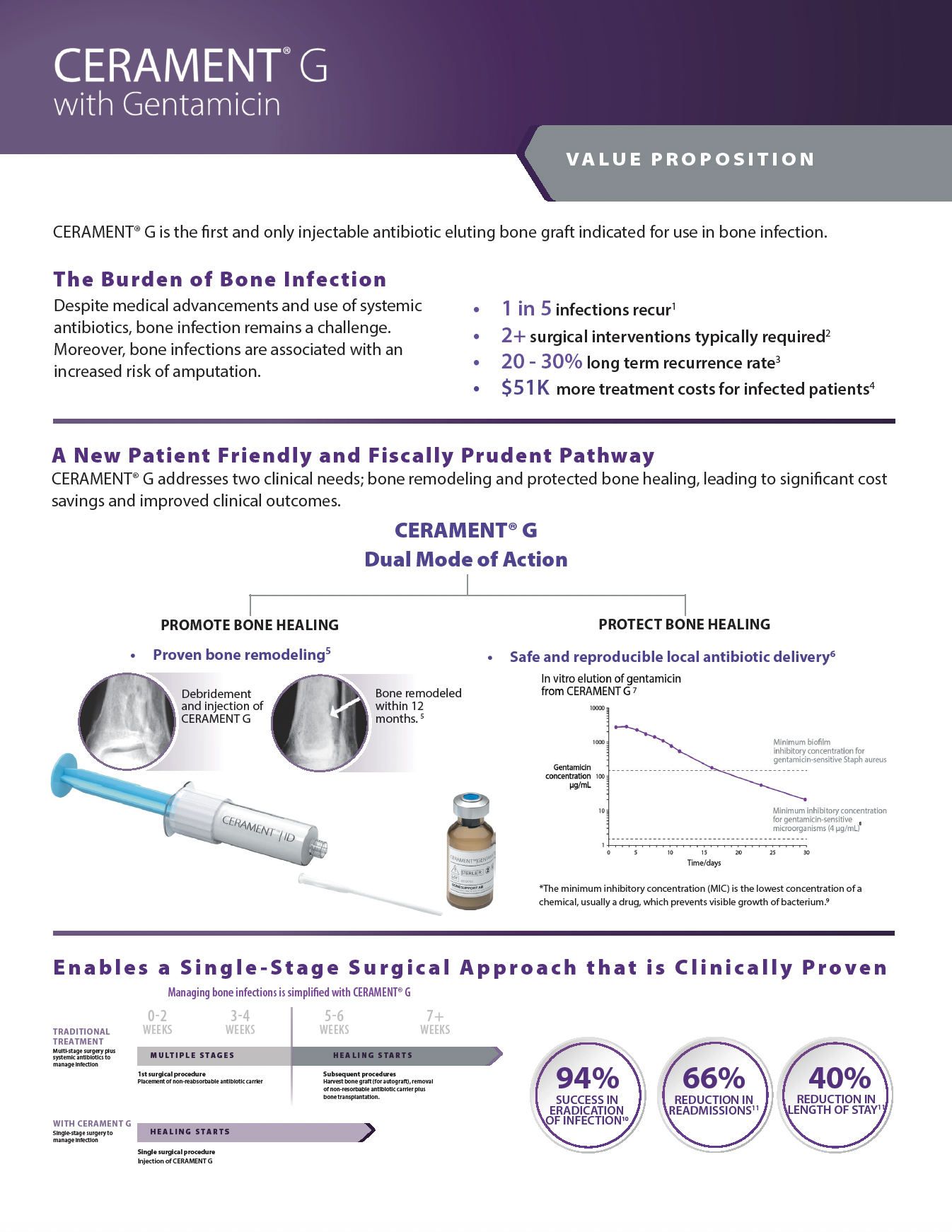
FIRST & ONLY:
FDA authorized, injectable, broad spectrum antibiotic-eluting bone void filler.
All in One:
Combined Antibiotic-Eluting Bone Graft
One & Done:
Promotes Bone Healing and Elutes Antibiotic Locally
CERAMENT G is an injectable synthetic bone void filler consisting of 40% hydroxyapatite, 60% calcium sulfate and the antibiotic gentamicin sulfate.
The unique ratio of hydroxyapatite and calcium sulfate is designed to enable CERAMENT to resorb at the same rate that bone forms. Calcium sulfate acts as a resorbable carrier for hydroxyapatite, and hydroxyapatite is highly osteoconductive, promoting bone ingrowth.
The addition of gentamicin protects against colonization by gentamicin-sensitive microorganisms. CERAMENT G is indicated for use in bone infection.
The characteristics of CERAMENT G mean that surgeons can manage bone defects in a more patient-friendly single-stage procedure. CERAMENT G has proven clinical outcomes, with one of the lowest (re)fracture and (re)infection rates of any synthetic bone substitute on the market. Moving to a single-stage from a multi-stage surgical protocol also frees healthcare resources to meet other needs, paving the way for a more cost-effective strategy for hospitals.
Remodels into host bone within 6-12 months
With CERAMENT, simultaneous de novo bone formation is seen throughout the material and it is fully remodeled into host bone within 6-12 months. Rapid bone remodeling reduces the risk of fracture, non-union and (re)infection.
Injectable and flowable
CERAMENT can be injected through a 16G needle, making it ideal for minimally invasive procedures.
Flowability ensures excellent spread into and around a bone defect, for complete dead space management. This is particularly important for the healing of complex defects, where the extent of bone loss may not be apparent at first and certain areas may be difficult to access.
Gentamicin elution above MIC that is reliable and consistent
To protect the CERAMENT implant from bacterial colonization, it is essential to have a high initial concentration of gentamicin to deliver the most effective bactericidal blow to the pathogens that could cause a bone infection and to maintain the gentamicin concentration above MIC levels for a clinically relevant period of time (>28 days).
High local concentration of gentamicin, without high serum gentamicin levels
CERAMENT G has been designed to offer high local concentrations of gentamicin to effectively protect bone healing from gentamicin-sensitive organisms.
The high local concentration of gentamicin is safe. In vivo studies have shown that CERAMENT G offers local gentamicin concentration levels 64-150 times higher than the minimum inhibitory concentration (MIC) for gentamicin-sensitive pathogens such as Staphylococcus aureus and Pseudomonas aeruginosa.
In patients with long bone osteomyelitis treated with up to 30 mL CERAMENT G (mean volume of 11.6 mL injected), the highest reported serum concentration of gentamicin was < 2 mg/mL within 24 hours after surgery, well below the gentamicin serum toxicity threshold level of 12 mg/mL.