The CERAMENT portfolio of products are synthetic bone void fillers consisting of 40% hydroxyapatite and 60% calcium sulfate.
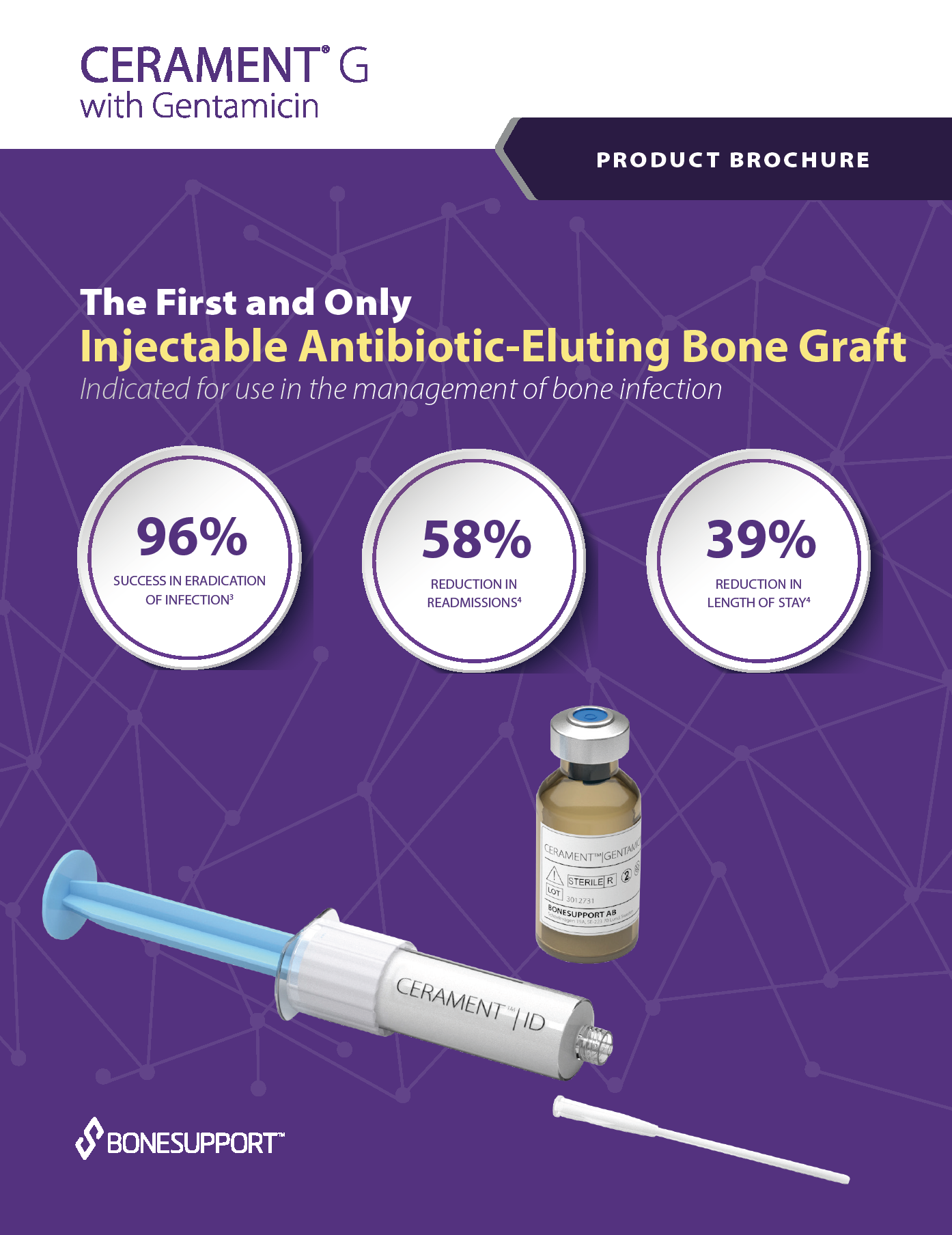
There are two products available; CERAMENT BONE VOID FILLER and CERAMENT G (with gentamicin).
All CERAMENT products are injectable through a 16G needle, flowable for complete bone defect filling, and remodel into bone with 6-12 months.
CERAMENT is effective in treating patients with fractures and bone voids caused by trauma, infection, disease or related surgery.
CERAMENT BONE VOID FILLER is ideal for patients with critical-sized bone defects where you would like to promote bone healing.
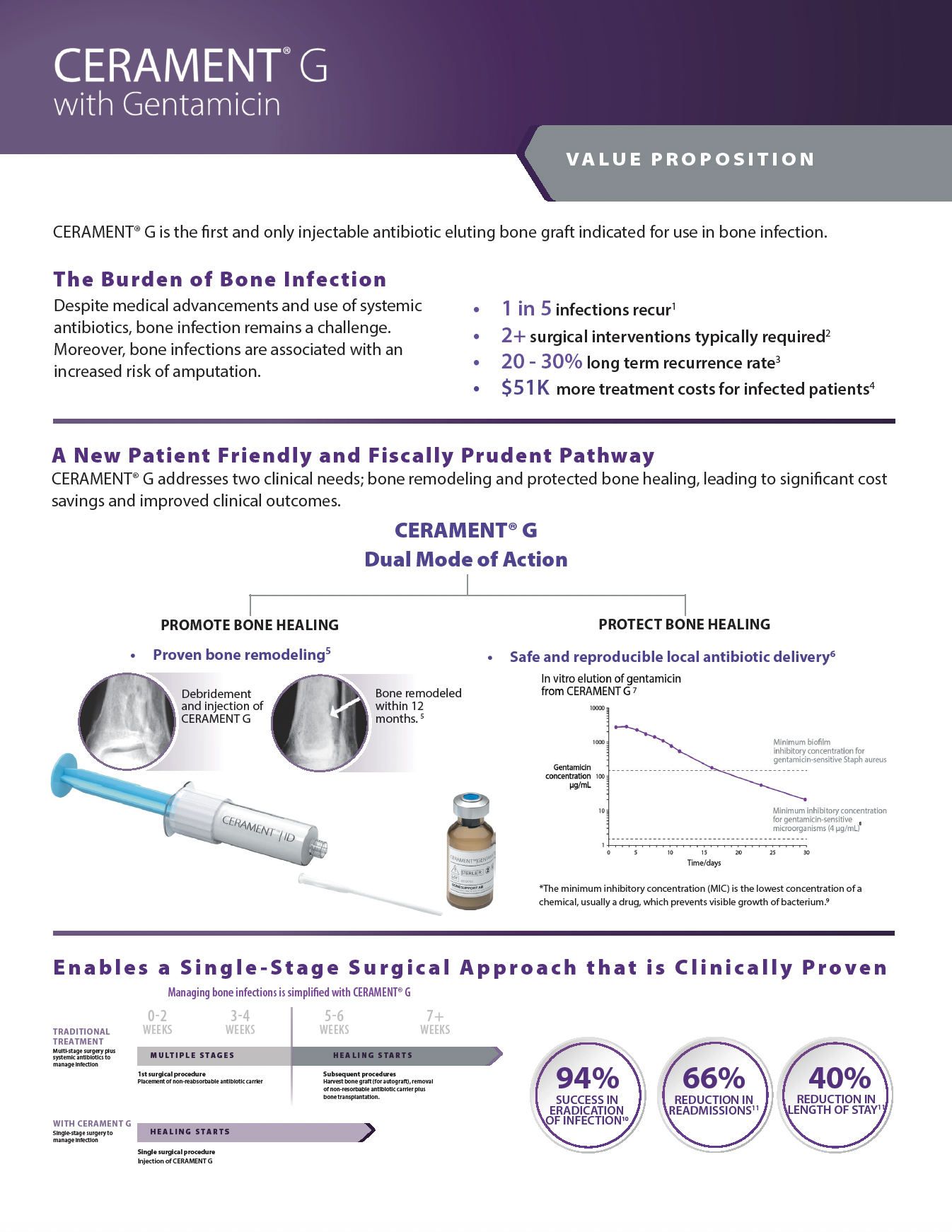
To protect bone healing in patients with infected bone defects, BONESUPPORT offers CERAMENT G with gentamicin.
The characteristics of CERAMENT G mean that surgeons can manage bone defects in a more patient-friendly single-stage procedure. CERAMENT G has proven clinical outcomes, with one of the lowest (re)fracture and (re)infection rates of any synthetic bone substitute on the market. Moving to a single-stage from a multi-stage surgical protocol also frees healthcare resources to meet other needs, paving the way for a more cost-effective strategy for hospitals.
There are more than 240+ publications and abstracts supporting the CERAMENT portfolio of products, and our key papers can be found on our publications and resources page.
We hold regular educational webinars, and all our upcoming and past webinars can be seen here.
If you have any comments, feedback or a complaint about our products, please contact us.