Removing Financial Barriers for Patients and Providers
CERAMENT® G with Gentamicin has been designated by the FDA as a “Breakthrough Device”. This designation has allowed easier access to reimbursement programs through the Centers for Medicare and Medicaid Services (CMS) for both the inpatient and outpatient settings. The purpose of these programs is to expand patient access to new technology that CMS deems as worth adopting and help ease the decisions between cost and care.
Outpatient Reimbursement Program
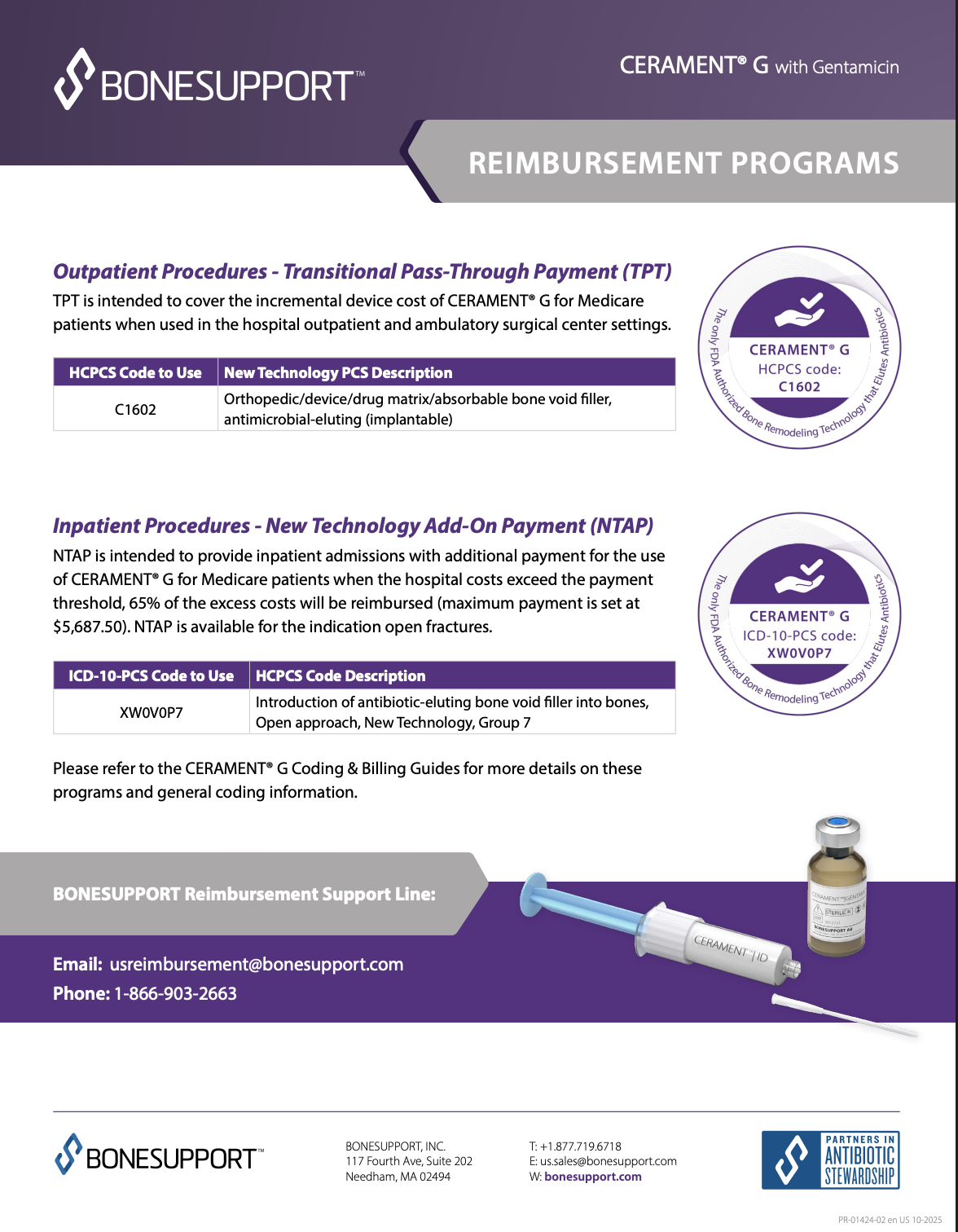
In the fall of 2023 CMS approved CERAMENT G for outpatient reimbursement through CMS’s Transitional Pass-Through payment. This payment is intended to reimburse hospital outpatient departments and ambulatory surgical centers for the incremental device cost of CERAMENT G. The purpose of this reimbursement program is to expand access to new technology and provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions than current standard of care/alternatives.
Transitional-Pass-Through Payment (TPT) is intended to cover the incremental device cost of CERAMENT G with Gentamicin, for Medicare patients, when used in the hospital outpatient and ambulatory surgical settings.
| HCPCS Code | HCPCS Code Description |
| C1602 | Orthopedic/device/drug matrix/absorbable bone void filler, antimicrobial-eluting (implantable) |
To learn more about our CERAMENT G with Gentamicin Outpatient Reimbursement Program, click here.
![]()
Inpatient Reimbursement Program
CERAMENT® G with Gentamicin has been designated by the FDA as a “Breakthrough Device”. This designation has allowed easier access to reimbursement programs through the Centers for Medicare and Medicaid Services (CMS) for both the inpatient and outpatient settings. The purpose of these programs is to expand patient access to new technology that CMS deems as worth adopting and help ease the decisions between cost and care.
In August of 2025 CMS approved CERAMENT G for a New Technology Add-On Payment for the indication open fractures. New Technology Add-On Payment (NTAP) is intended to provide inpatient admissions with additional payment for the use of CERAMENT G with Gentamicin when the hospital costs exceed the payment threshold. Payment varies based on costs exceeding the payment threshold with a max payment of $5,687.50.
The case must be coded with the ICD-10-PCS procedure XW0V0P7 (i.e., CERAMENT G was used in the surgery), but without any of the ICD-10-CM diagnosis codes in the category M86 (Osteomyelitis). Those cases with an M86 code do not qualify for this NTAP.
| ICD-10-PCS Code | ICD-10-PCS Code Description |
| XW0V0P7 | Introduction of antibiotic eluting bone void filler into bones, Open Approach, New Technology Group 7 |
Note: During October 2023 to September 2025, CERAMENT G had an active NTAP for the indication bone infection. The NTAP for the first indication was discontinued after three years’ duration.
![]()